Predicting Precipitates with Solubility Rules
11th Grade · Chemistry · 60 min
Lesson Preview
Learning Objective
I can use a Solubility chart to predict whether a precipitate will form in an aqueous solution.
- 1
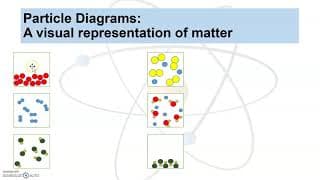
A solubility table helps determine if a compound is soluble (aqueous) or insoluble (solid).
- 2
Group 1 metals, ammonium, nitrate, and acetate are generally soluble with no exceptions.
- 3
Halogens are typically soluble, except when combined with silver, lead, or mercury, which makes them insoluble.